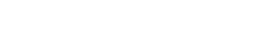E. Mycotoxins
Mycotoxins are toxic compounds produced by fungi that occur naturally in grains. When consumed at elevated levels, mycotoxins may cause sickness in animals and humans. While several mycotoxins have been found in corn grain, aflatoxins and deoxynivalenol (DON or vomitoxin) are considered to be two of the important mycotoxins.
The U.S. grain merchandising industry implements strict safeguards for handling and marketing any elevated levels of mycotoxins. All stakeholders in the corn value chain – seed companies, corn growers, grain marketers and handlers as well as U.S. corn export customers – are interested in understanding how mycotoxin infection is influenced by growing conditions and the subsequent storage, drying, handling and transport of the grain as it moves through the U.S. corn export system.
To assess the impact of these conditions on aflatoxins and DON development, this report summarizes the results from official USDA Federal Grain Inspection Service (FGIS) aflatoxin tests and from independent DON tests for all the export samples collected as part of this survey. Details on the testing methodology employed in this study for the mycotoxins are in the “Testing Analysis Methods” section.
1. Aflatoxins Testing Results
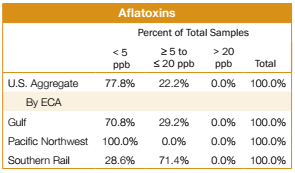
FGIS tested all 397 samples for the Export Cargo Report 2012/13 for aflatoxins. Results of the 2012/13 survey are as follows:
- 309 or 77.8%, of the samples had no detectable levels of aflatoxins (less than 5.0 ppb, the FGIS lower reporting level). For the 2011/12 report, 75.2% of the samples tested had no detectable levels of aflatoxins.
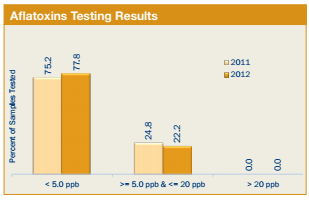
- 88 samples or 22.2%, of the samples showed aflatoxins in levels greater than or equal to 5.0 ppb but less than the FDA action level of 20 ppb, compared to 24.8% of the samples tested for the 2011/12 exports.
- 100% of the export samples tested were below or equal to the FDA action level of 20 ppb, the same percentage as for the 2011/12 export samples tested. 2. DON (Deoxynivalenol or Vomitoxin) Testing Results All 397 samples for the Export Cargo Report 2012/13 were tested for DON. Results of the testing are shown below:
2. DON (Deoxynivalenol or Vomitoxin) Testing Results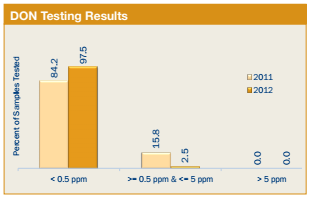
All 397 samples for the Export Cargo Report 2012/13 were tested for DON. Results of the testing are shown below:
- 387 samples (97.5%) had less than 0.5 ppm of DON compared to 84.2% of the 2011/12 export samples.
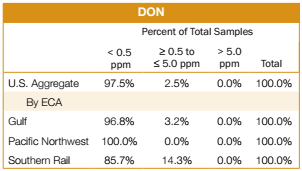
- 10 samples, or 2.5% of the samples, tested greater than or equal to 0.5 ppm, but less than or equal to the FDA advisory level of 5 ppm. For the 2011/12 export samples, 15.8% of the samples had detectable DON levels in the same range.
- 100% of the samples tested below or equal to the FDA advisory level of 5 ppm, the same percentage as for the 2011/12 export samples.
3. Mycotoxin Background: General
The levels at which the fungi produce mycotoxins are influenced by the fungus type and the environmental conditions under which the corn is produced and stored.
Because of these differences, mycotoxin production varies across the U.S. corn-producing areas and across years.
Humans and livestock are sensitive to mycotoxins at varying levels. As a result, the U.S. Food and Drug Administration (FDA) has issued action levels for aflatoxins and advisory levels for DON by intended use.
Action levels specify precise limits of contamination above which the agency is prepared to take regulatory action. Action levels are a signal to the industry that FDA believes it has scientific data to support regulatory and/or court action if a toxin or contaminant is present at levels exceeding the action level if the agency chooses to do so. If import or domestic feed supplements are analyzed in accordance with valid methods and found to exceed applicable action levels, they are considered adulterated and may be seized and removed from interstate commerce by FDA.
Advisory levels provide guidance to the industry concerning levels of a substance present in food or feed that are believed by the agency to provide an adequate margin of safety to protect human and animal health. While FDA reserves the right to take regulatory enforcement action, enforcement is not the fundamental purpose of an advisory level.
A source of additional information is the National Grain and Feed Association (NGFA) guidance document titled “FDA Regulatory Guidance for Toxins and Contaminants” found at http://www.ngfa.org/files/misc/Guidance_for_Toxins.pdf.
4. Mycotoxin Background: Aflatoxins
The most important type of mycotoxin associated with corn grain is aflatoxin. There are several types of aflatoxin produced by different species of the Aspergillus fungus with the most prominent species being A. Flavus. Growth of the fungus and aflatoxin contamination of grain can occur in the field prior to harvest or in storage. However, contamination prior to harvest is considered to cause most of the problems associated with aflatoxin. A. Flavus grows well in hot, dry environmental conditions or where drought occurs over an extended period of time. It can be a serious problem in the southern United States where hot and dry conditions are more common. The fungus usually attacks only a few kernels on the ear and often penetrates kernels through wounds produced by insects. Under drought conditions, it also grows down silks into individual kernels.
The FDA has established action levels for aflatoxin in milk intended for human consumption and for total aflatoxins in human food, grain and livestock feed products (see table below).
FDA has established additional policies and legal provisions concerning the blending of corn with levels of aflatoxins exceeding these threshold levels. In general, FDA currently does not permit the blending of corn containing aflatoxins with uncontaminated corn to reduce the aflatoxin content of the resulting mixture to levels acceptable for use as human food or animal feed.
Corn exported from the U.S. must be tested for aflatoxins according to Federal law. Unless the contract exempts this requirement, testing must be conducted by FGIS. Corn above the FDA action level of 20 ppb cannot be exported unless other strict conditions are met. These requirements result in relatively low levels of aflatoxins in exported grain.
5. Mycotoxin Background: DON (Deoxynivalenol) or Vomitoxin
DON is another mycotoxin of concern to some importers of corn grain. It is produced by certain species of Fusarium, the most important of which is F. graminearum (Gibberella zeae) which also causes Gibberella ear rot (or red ear rot). The fungus can be spotted easily in corn because of the conspicuous red discoloration of kernels on the ear. The presence of Gibberella zeae is mostly a problem when warm, wet weather occurs at flowering. The fungus grows down the silks into the ear, and in addition to producing DON, it results in damage to kernels that are evident during the grain inspection process. DON and Gibberella ear rot are most common in the northern Corn Belt states. This may be due to the susceptibility to the fungus of very early maturing corn hybrids commonly grown in these areas.
DON is mostly a concern with monogastric animals where it may cause irritation of the mouth and throat.
As a result, the animals may eventually refuse to eat the DON-contaminated corn and may have low weight gain, diarrhea, lethargy, and intestinal hemorrhaging. It may cause suppression of the immune system resulting in susceptibility to a number of infectious diseases.
The FDA has issued advisory levels for DON. For products containing corn, the advisory levels are:
- 5 ppm in grains and grain by-products for swine, not to exceed 20% of their diet,
- 10 ppm in grains and grain by-products for chickens and cattle, not to exceed 50% of their diet, and
- 5 ppm in grains and grain by-products for all other animals, not to exceed 40% of their diet.
FGIS is not required to test for DON on corn bound for export markets, but will perform either a qualitative or quantitative test for DON at the buyer’s request.